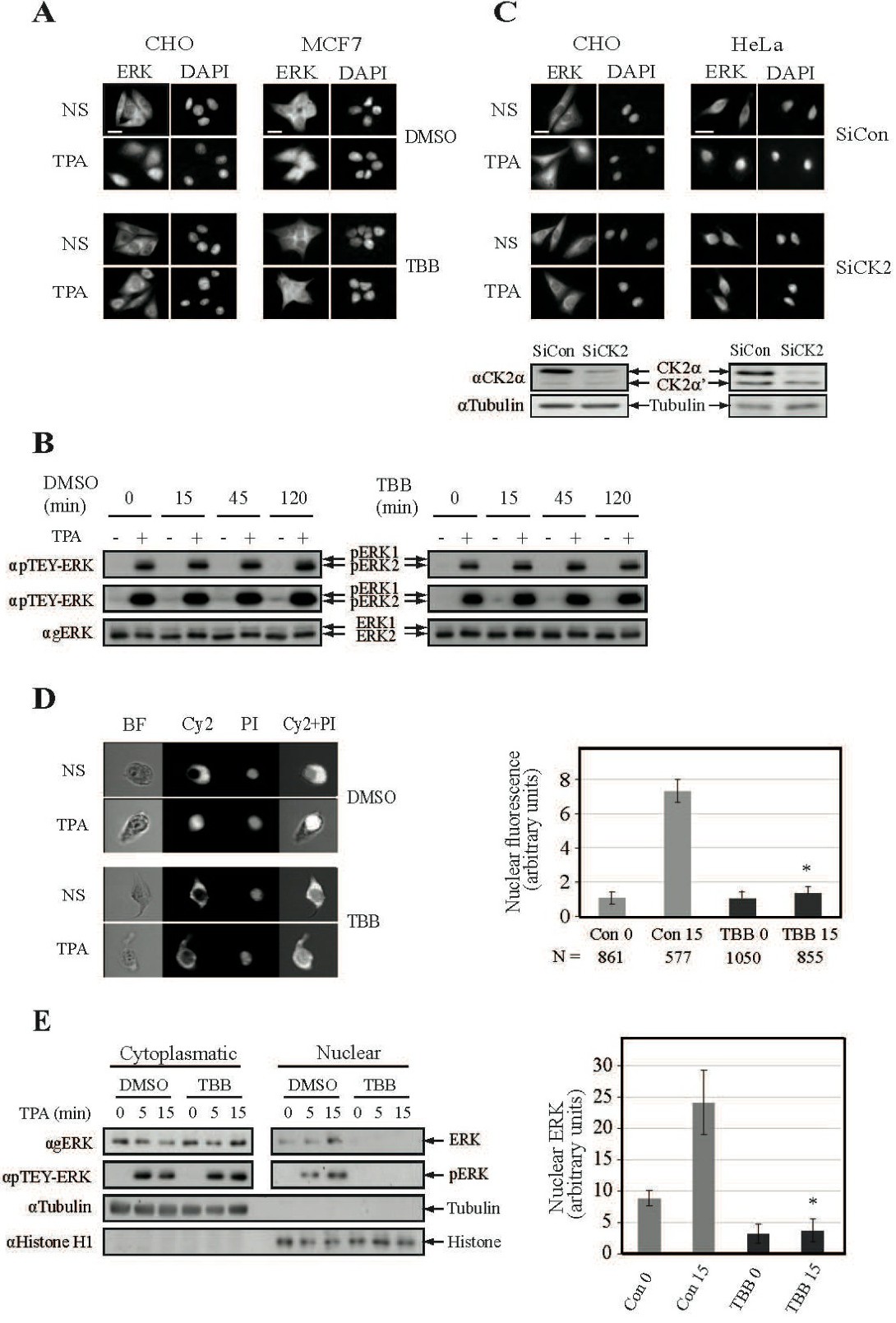
Fig. 1. CK2 plays a role in the nuclear translocation of ERK1/2. (A) CK2 inhibition prevents nuclear translocation of endogenous ERK1/2. CHO and MCF7 cells were serum-starved (16 h, 0.1%), pretreated with either TBB (10 mM, 2 h) or DMSO control, and then either stimulated with TPA (250nM, 15 min) or left untreated as control. The cells were stained with agERK1/2 Ab and DAPI. Scale-bar - 15 Ám. (B) TBB does not affect TEY-ERK phosphorylation. HeLa cells were serum starved, pretreated with TBB (10μM) or DMSO ( indicated times) and then stimulated with TPA (250nM, 15 min). Cell extracts were subjected to Western blot analysis using αpERK (low exposure and high exposure) and αgERK Abs. (C) Knockdown of CK2 prevents nuclear translocation of endogenous ERKs. CHO and HeLa cells were transfected with either siCK2a or non-relevant siRNA (siCon). Thirty-six h after transfection the cells were serum starved, stimulated and processed as above. The efficiency of CK2a knockdown is shown as compared to endogenous tubulin levels. (D) Analysis of CK2 effect on the nuclear translocation of endogenous ERK1/2 by ImageStream. HeLa cells were serum and treated as above, and then the cells were analyzed by ImageStream. The graph represents fluorescence of nuclear ERK1/2 (two independent experiments), where the fluorescence of non-stimulated control was considered as 1. N - number of cells analyzed. * - P (student T-test) <0.02. (E) Cell fractionation confirms the CK2's role. HeLa cells were treated as in 1A. After fractionation, cytoplasmic and nuclear samples were subjected to Western blot using the indicated Abs. Tubulin and Histone H1 served as localization controls. The graph represents densitometric analysis of nuclear ERK1/2 (two independent experiments). * - P <0.05.